Industry leader in reformulating solid drugs into oral suspensions to improve drug absorption and better meet patients' needs


PIPELINE
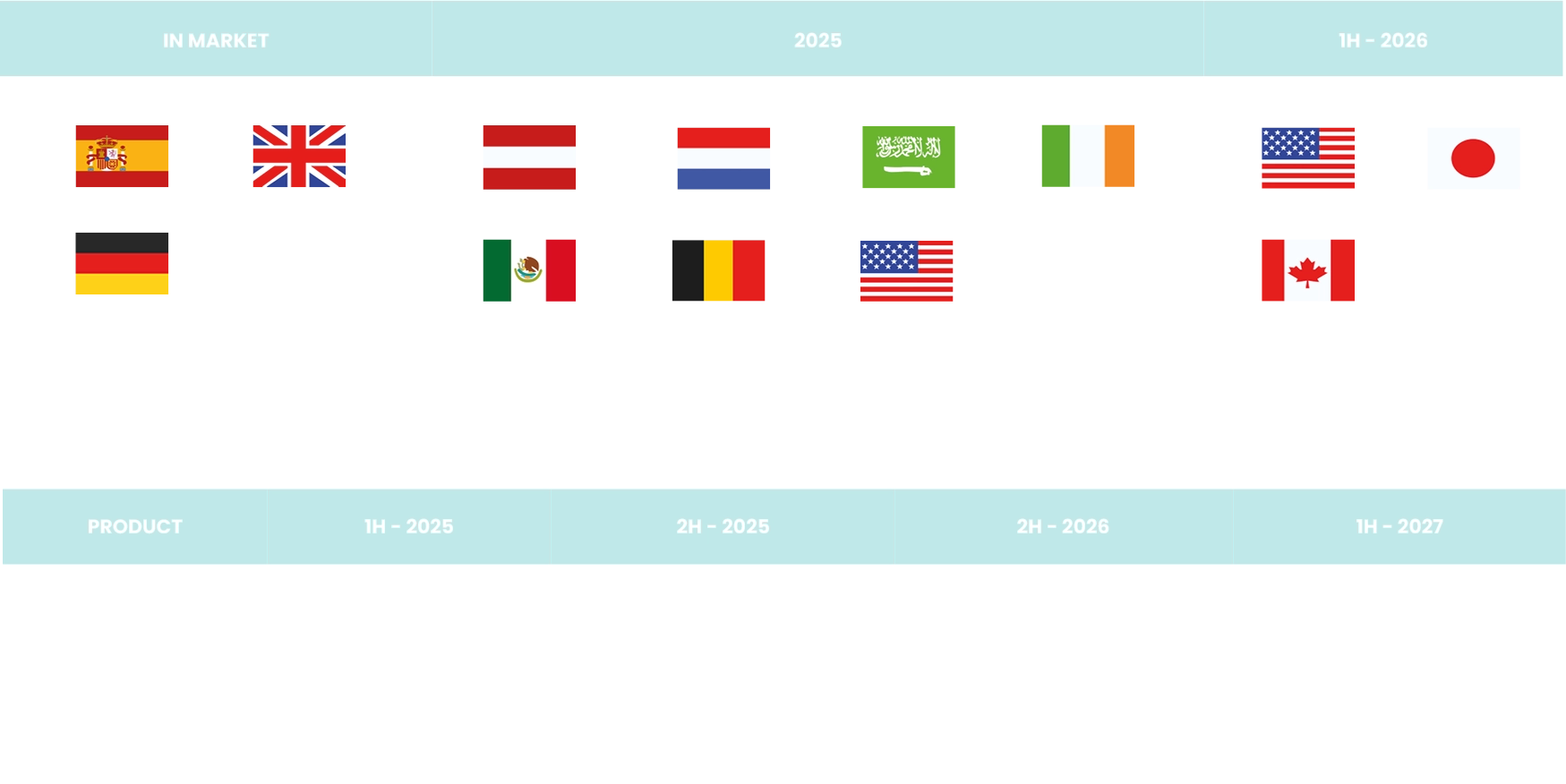
The company’s development pipeline includes multiple programs focused in the Rx, OTC and electronic medical device sector.
Our approach centers on the reformulation and development of products, solving chronic to emergent conditions; from diabetes medications to more standard treatments such as pain relief and GI conditions. The below charts represent our anticipated launch dates.
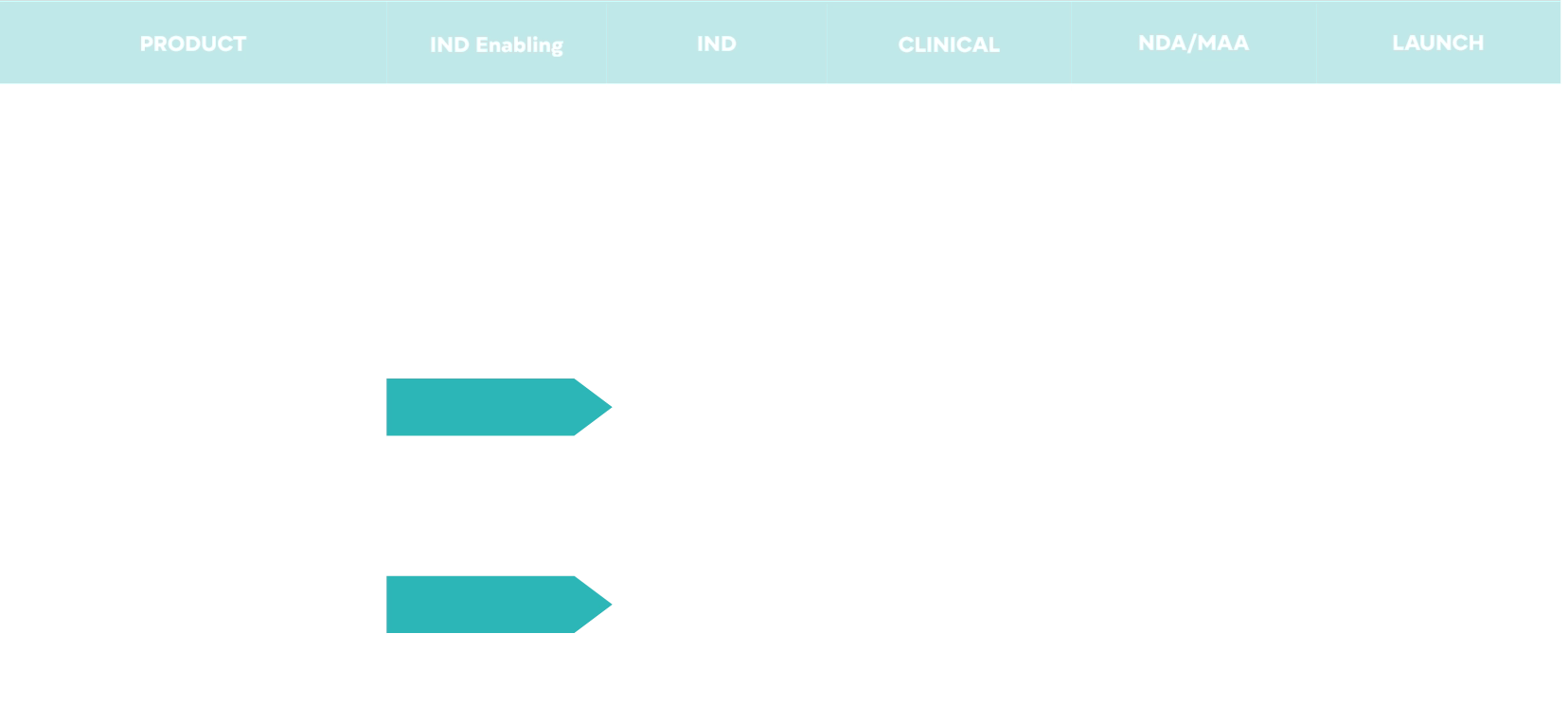
RX Products
Significant development of critical Rx prodcuts is currently underway. The anticipated road map is below. We will address multiple indications including early work within oncology.
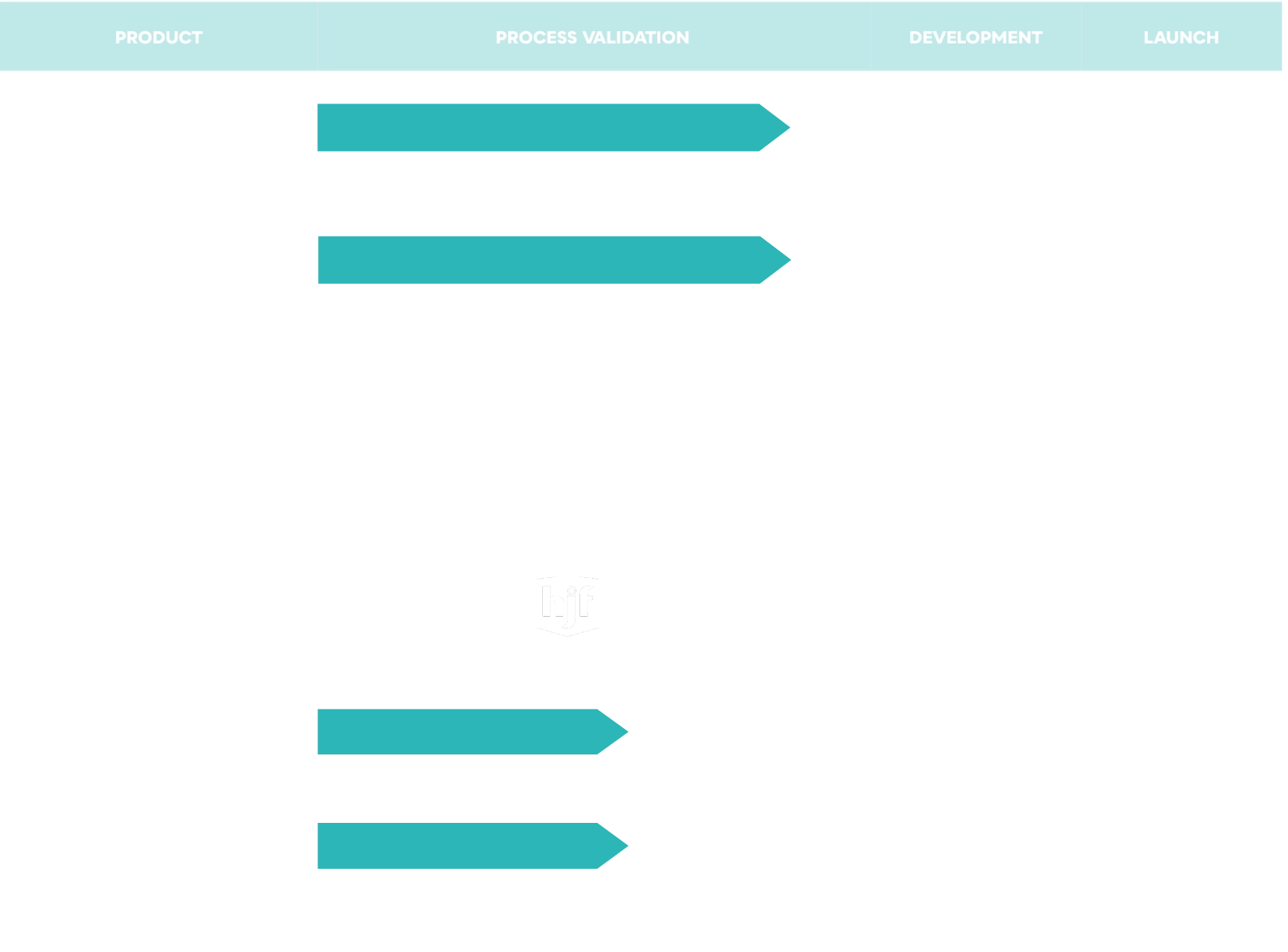
OTC Products
The reformulation of well known and important products will continue to bolster Aspargo Labs product portfolio. The anticipated road map is below.
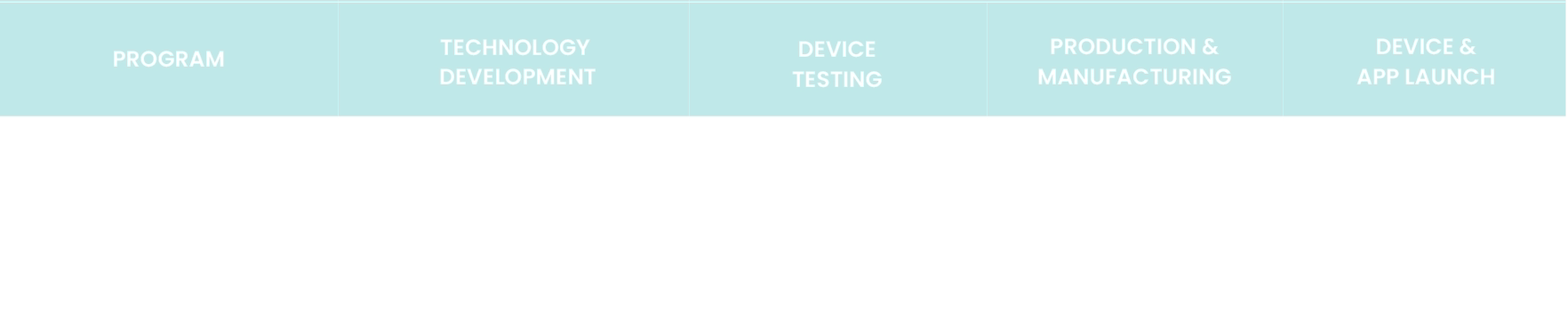
Device Development
The creation of a next generation user-friendly oral medicine delivery device with mobile connectivity software. It will enable precise dosing and encourage adherence while providing real time patient data to healthcare professional and caregivers. The anticipated road map is below.