Converting the World’s Most Meaningful Medications into
Faster Acting and More Concentrated
Liquid Oral Suspensions





ORAL SUSPENSION TECHNOLOGY
Improving Patient Experience
Aspargo Labs’ oral suspension products enhance patient experiences and empower physicians to manage health outcomes more effectively. Administered orally, our formulations achieve a higher absorption rate.
Our unique suspension technology optimizes the interplay between molecular particle size, coating, and viscosity, resulting in more efficient treatment delivery.
DEVELOPMENT
Setting the Standard for Patient Centric Care

Suspension Technology
Oral suspension formulations have several advantages including accelerated absorption rates, higher bioavailability, better taste, and are often preferred by patients who have difficulty swallowing.
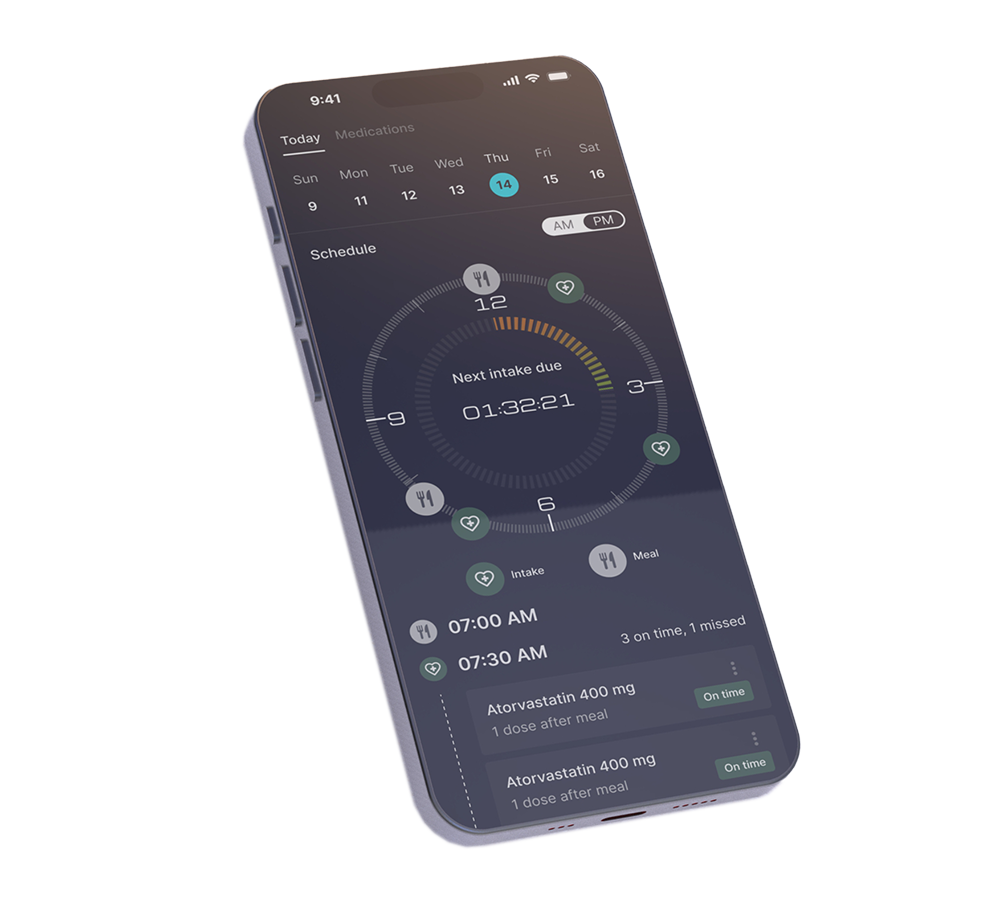
User-Friendly Mobile Platform
Aspargo Labs is at the forefront of developing a high-tech device with replaceable medication cartridges, which in the future will also be connected to a user-friendly mobile platform.
Treatment Options
These cartridges will be available for multiple medications across various therapeutic areas, and the device will provide real time data to benefit patients, physicians and caregivers.

DEVELOPMENT
Setting the Standard for Patient Centric Care

Suspension Technology
Oral suspension formulations have several advantages including accelerated absorption rates, higher bioavailability, better taste, and are often preferred by patients who have difficulty swallowing.

User-Friendly Mobile Platform
Aspargo Labs is at the forefront of developing a high-tech device with replaceable medication cartridges, which in the future will also be connected to a user-friendly mobile platform.

Treatment Options
These cartridges will be available for multiple medications across various therapeutic areas, and the device will provide real time data to benefit patients, physicians and caregivers.
BENEFITS
Treatment benefits of suspension technology:

Improved Absorption
Optimizing the bioavailability of active ingredients in suspension technology for more efficient uptake

No Need for Planning
Liquid formulations reduce the complexity of dosing schedules

Precise Meter Dosing
Allows flexibility to personalize dose regimens

Adherence
Ease of administration helps patients follow doctors recommendations leading to more consistent use

Easier to Swallow
Almost 40% of the population report having difficulty to swallow pills, especially for pediatric and geriatric populations
GLOBAL ACCOMPLISHMENTS
Our impact through groundbreaking achievements & innovation
Thanks to our proprietary technology, Aspargo Labs launched a liquid oral sildenafil suspension product (ASP-001) the active ingredient in Viagra®.
Product is currently marketed under the brand names of HEZKUE® in Germany, UK and Ireland and BANDOL® in Spain.
ASP-001 has also received regulatory approval in additional countries: Mexico, Netherlands and Argentina.
Aspargo Labs has completed all clinical trails to satisfy the submission of an NDA with the FDA.

Additional oral suspensions are in development for both Rx and OTC products.










