Adds experienced public-company finance executive to advance Aspargo’s corporate, commercial, and capital markets strategies
NEW YORK, Feb. 18, 2026 (GLOBE NEWSWIRE) – Aspargo Labs, Inc. (“Aspargo Labs” or the “Company”), a pharmaceutical company commercializing a highly absorbent oral suspension spray technology capable of achieving rapid therapeutic exposure, today announced the appointment of Sam Backenroth as Chief Financial Officer. The appointment further strengthens the company’s executive leadership as it enters its next phase of corporate growth and commercial expansion.
Mr. Backenroth brings close to 20 years of senior financial and strategic leadership experience in the biotechnology sector, including serving as CFO for multiple public and private companies across therapeutic areas such as ophthalmology, neurology, immunology, and oncology. He has supported companies in accessing and navigating the public markets, driving significant enterprise value creation and the execution of institutional capital raises. His scope of work includes capital markets transactions, public company reporting, investor engagement, and building scalable financial and governance infrastructure to support commercialization, operational growth, and long-term shareholder value creation.
“Sam has repeatedly demonstrated the ability to position companies for the expectations and rigor of the public markets,” said Michael Demurjian, Chief Executive Officer of Aspargo. “He brings deep expertise building institutional-grade financial infrastructure while maintaining strategic flexibility. His long-standing relationships within the investment banking and institutional investor communities, combined with his disciplined approach to financial execution and commercial preparation, will be highly valuable as we execute on both our near-term priorities and Aspargo’s long-term growth objectives.”
Throughout his career, Mr. Backenroth has worked closely with boards and executive teams to align financial and corporate strategy, capital planning, and external engagement through key corporate milestones and value inflection points. He will partner closely with the executive team to drive Aspargo’s ongoing U.S. and European product launches, support operational scaling, and advance new product development initiatives. His role will include aligning financial planning, capital strategy, and external communications with the company’s expanding commercial footprint.
“I’m excited to be joining Aspargo at a pivotal moment in its growth,” said Sam Backenroth, Chief Financial Officer at Aspargo Labs. “This platform has the potential to bring best-in-class products to patients at scale, and I look forward to translating that potential into sustainable growth while driving innovation and long-term value for all stakeholders.”
The appointment reflects Aspargo’s continued focus on strengthening its leadership, governance, and financial foundation in support of future opportunities.
About Aspargo Labs, Inc.
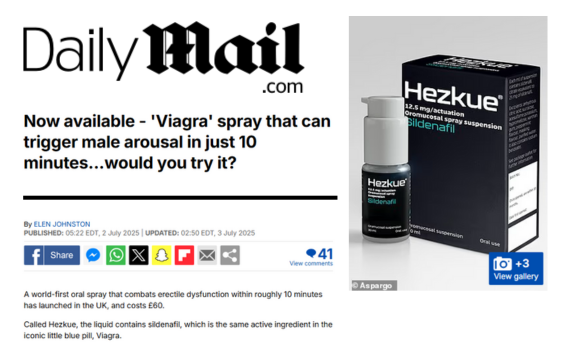
Aspargo Labs, Inc., is a pharmaceutical company led by an experienced team of drug developers focused on enhancing the delivery of the world’s most meaningful medications through highly absorbent liquid oral suspension sprays. The Company’s proprietary platform applies advanced pharmaceutical principles to accelerate the path to commercialization of products utilizing known active pharmaceutical ingredients. Aspargo’s oral suspension platform is custom designed to optimize each molecule’s unique properties to better align with patient needs, achieving rapid therapeutic exposure while offering key advantages including improved absorption, ease of administration, flexible dosing, and enhanced patient adherence. The Company is leveraging its integrated development platform across multiple therapeutic areas, beginning with a liquid oral sildenafil suspension spray currently marketed by Aspargo in multiple countries.
For additional information, please visit our website at www.aspargolabs.com.
FORWARD-LOOKING STATEMENTS
All statements other than statements of historical facts contained in this press release are forward-looking statements. Forward-looking statements reflect management’s current knowledge, assumptions, judgment, and expectations regarding future performance or events. Although management believes that the expectations reflected in such statements are reasonable, they give no assurance that such expectations will prove to be correct, and you should be aware that actual events or results may differ materially from those contained in the forward-looking statements. Words such as “will,” “expect,” “intend,” “plan,” “potential,” “possible,” “goals,” “accelerate,” “continue,” and similar expressions identify forward-looking statements.
Forward-looking statements are subject to a number of risks and uncertainties including, but not limited to, the risks inherent in our lack of profitability and need for additional capital; our dependence on partners to further the development of our product candidates; the uncertainties inherent in the development, attainment of the requisite regulatory authorizations and approvals and launch of any new pharmaceutical product; and the outcome of pending or future litigation or arbitration.
All forward-looking statements are expressly qualified in their entirety by this cautionary notice. You should not rely upon any forward-looking statements as predictions of future events. We undertake no obligation to revise or update any forward-looking statements made in this press release to reflect events or circumstances after the date hereof, to reflect new information or the occurrence of unanticipated events, or to update the reasons why actual results could differ materially from those anticipated in the forward-looking statements, in each case, except as required by law.
For more information:
Aspargo Labs, Inc.
(646) 503-1260
information@aspargolabs.com
Investor & media contacts:
Russo Partners
Nic Johnson or Liz Phillips
(347) 956-7697
aspargoIR@russopr.com